Leadless Pacemaker Systems
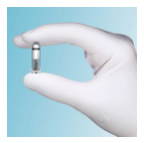 In April of 2016, the newest innovation in pacemaker therapy was approved in the United States – the Medtronic MICRA transvenously delivered leadless pacemaker system.
In April of 2016, the newest innovation in pacemaker therapy was approved in the United States – the Medtronic MICRA transvenously delivered leadless pacemaker system.
Leadless pacemaker systems no longer involve the need for a pacemaker generator to be placed in the chest along with leads running into the heart – it is directly implanted into the patient’s heart with no transvenous leads involved. This device is 93% smaller than a conventional pacemaker system.
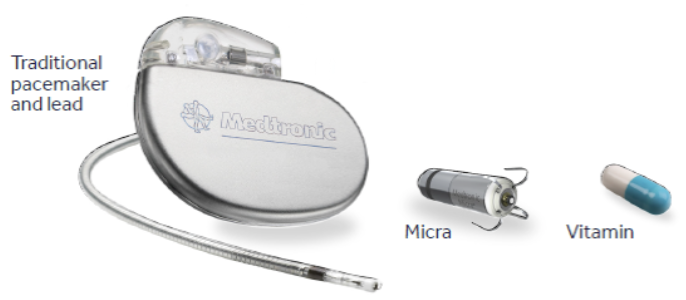
Figure 1. Image of conventional transvenous pacemaker system compared to the new MICRA leadless pacemaker system
This technique is less invasive and involves using a transvenous catheter delivery system via the patient’s femoral vein to insert a self-contained pacemaker system into the heart thus eliminating the need and complications arising from chest incision and wires running into the heart from the upper body venous system as with a conventional pacemaker system.
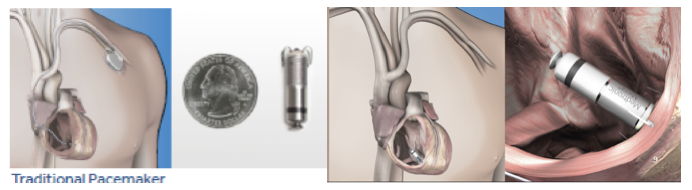
Figure 2. Comparison of traditional transvenous pacemaker system with MICRA leadless system – comparable to the size of a quarter- now placed in the bottom right ventricle of the heart with tines securing its position without the need for a wired system and traditional pacemaker placed in the upper chest.
The new leadless pacemaker system is generally safe for use with conventional MRI systems and has an estimated battery longevity on average of 12 years. As with conventional pacemaker systems, household appliances are safe to use. As well, patients can safely go through airport security metal detectors with this device with minimal concern.
The new MICRA pacemaker system is not intended to replace the conventional transvenous pacemaker system and you will need to discuss with your physician if you are a good candidate for this single-chamber ventricular pacemaker system. Indeed, the current MICRA system is only available as a single-chamber pacemaker device thus not applicable to those patients who need a dual-chamber system.
VIDEO: How the leadless pacemaker system MICRA is implanted




 Silver Spring Office
Silver Spring Office  DC Office (at Providence Hospital)
DC Office (at Providence Hospital)  Hagerstown Office
Hagerstown Office