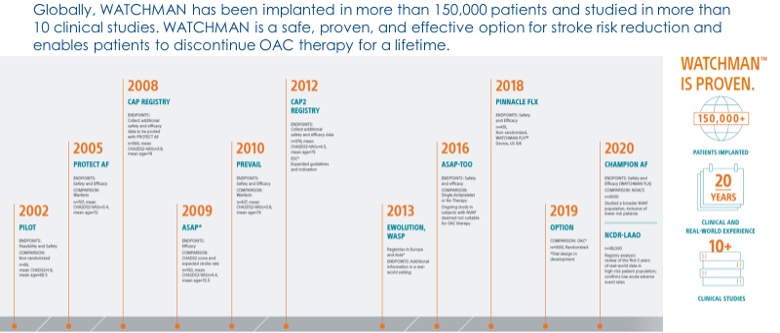
WATCHMAN – Left Atrial Appendage Occlusion Device
With the original FDA approval in 2015 for the WATCHMAN device along with now the second-generation WATCHMAN FLX left atrial appendage occlusion device, over 150,000 patients have been successfully treated since 2002.
The WATCHMAN FLX device is indicated to reduce stroke risk in patients with nonvalvular A-fib who require an alternative to long-term oral anticoagulation and per guidelines are at high enough risk for thromboembolic events that anticoagulation is already recommended.
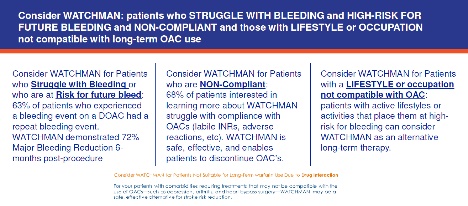
Atrial fibrillation creates an environment for thrombus formation in the left atrium, hence increasing the risk of thromboembolic events in those patients with increased risk factors. The WATCHMAN FLX device is a permanent implant designed to close the left atrial appendage in the heart in an effort to reduce the risk of stroke.
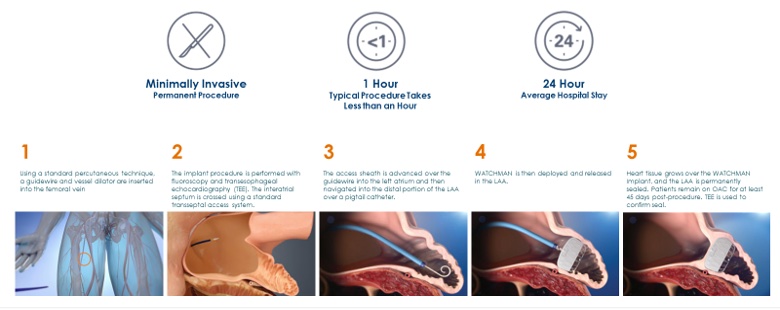
With all medical procedures there are risks associated with the implant procedure and the use of the device. The risks include but are not limited to accidental heart puncture, air embolism, allergic reaction, anemia, anesthesia risks, arrhythmias, AV (Arteriovenous) fistula, bleeding or throat pain from the TEE (Trans Esophageal Echo) probe, blood clot or air bubbles in the lungs or other organs, bruising at the catheter insertion site, clot formation on the device, cranial bleed, excessive bleeding, gastrointestinal bleeding, groin puncture bleed, hypotension, infection/pneumonia, pneumothorax, pulmonary edema, pulmonary vein obstruction, renal failure, stroke, thrombosis and transient ischemic attack. In rare cases death can occur.
Be sure to talk with your doctor so that you thoroughly understand all of the risks and benefits associated with the implantation of the device.




 Silver Spring Office
Silver Spring Office  DC Office (at Providence Hospital)
DC Office (at Providence Hospital)  Hagerstown Office
Hagerstown Office