Atrial Fibrillation
Overview
Atrial fibrillation (AF) is the most common cardiac arrhythmia and affects nearly 1% of the general population. Its prevalence increases with age, and occurs in up to 5% of those over 80 years of age. The normal pacemaker of the heart originates from the sinus node, a region in the upper wall of the top right atrium. It typically fires at a rate of 60 to 90 beats per minute, with depolarization and contraction of both the left and right atrium synchronized before contraction of their respective bottom ventricles. The electrical signal travels, as discussed in earlier sections, from the atria to the AV node, the “traffic control” center. It is here that the electrical signal is slightly delayed before it traverses to the bottom ventricles via the specialized conduction system of the bundle branchesand terminal purkinje fibers (discussed in the “Your Heart” section).
During AF, the atria can contract anywhere from 400-600 beats per minute, in an irregular and chaotic fashion. Fortunately, the AV node, the “traffic control” center, prevents 1 to 1 conduction to the ventricles. It may allow every 2-3 atrial beats to pass to the ventricles in an irregular fashion. The resultant heartbeat on exam is typically fast, but with medications, it can be slow with an irregular pulse.
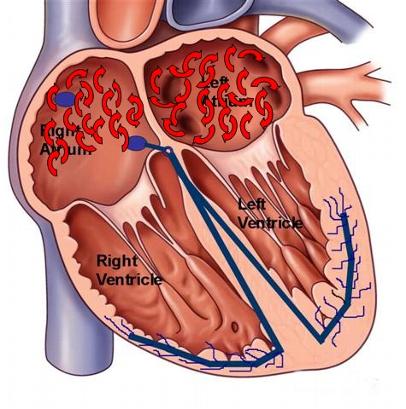
Figure 1. Drawing of the heart with wavelets of atrial depolarization and chaotic activity typical of atrial fibrillation
Atrial fibrillation can occur in patients with no structural heart disease (lone AF), typically seen in the younger population of patients. However, cardiac disorders such as coronary artery disease, congestive heart failure, and valvular heart disease can predispose patients to atrial fibrillation.
Causes
At the basic level, AF is caused by one or more premature atrial depolarizations originating from either the right or left atrium. These premature beats occur before the atria are expecting it, at a somewhat vulnerable period that may provoke or trigger AF. It is the origin of these premature atrial beats that have been the focus of the latest research into AF. In addition to premature atrial beats, a prolonged run of another atrial tachyarrhythmia, including atrial tachycardia (AT), atrial flutter, AV reciprocating tachycardia (AVRT), or even AV nodal reentrant tachycardia (AVNRT) that leads to repetitive fast atrial stimulation can lead to paroxysms of AF. Elimination of these premature atrial beats or atrial tachyarrhythmias with medication or catheter ablation has proven effective as a therapy to potentially “cure” paroxysmal AF. It is in this respect that the newest treatment modalities for AF have been tailored. It has been demonstrated that the left atrial pulmonary veins have residual left atrial tissue invading the cuff of the veins. It is from this tissue, that atrial premature beats and repetitive atrial tachycardias can arise, leading to the induction and perpetuation of clinical AF. Techniques for catheter ablation of AF have been developed to target and electrically isolate the pulmonary veins with the delivery of radiofrequency energy via an ablation catheter.
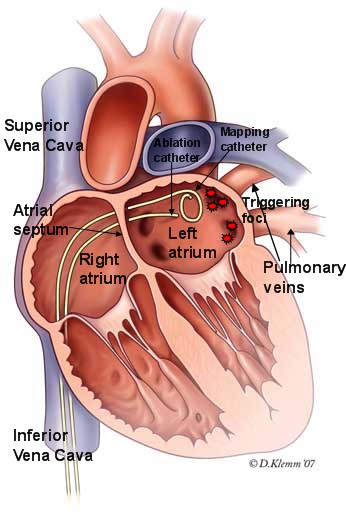
Figure 2. Illustration of technique to isolate the pulmonary veins in catheter ablation procedure for patients with atrial fibrillation. The ablation catheter is advanced to the left atrium via a puncture across the atrial septum. Once in the left atrium, the ablation catheter is maneuvered to the area of origin of the pulmonary veins responsible for the triggering foci. Radiofrequency energy is delivered to electrically isolate each of the pulmonary veins in this procedure.
The published success rates at different institutions for preventing clinical recurrences in the paroxysmal AF subgroup ranges from 70-80%. Not every patient is an appropriate candidate for a catheter-based ablation procedure, and you should consult your heart rhythm specialist for an individual tailored approach.
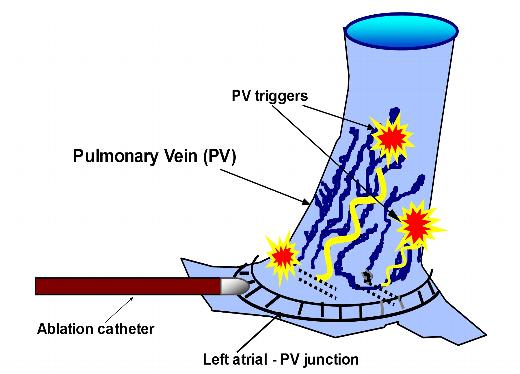
Figure 3. Schema of the left atrial-pulmonary vein ostium. Muscular extensions of the left atrial tissue into the pulmonary veins may serve as the originating foci of premature atrial beats and pulmonary vein atrial tachycardias. It is these foci that lead to the initiation and perpetuation of atrial fibrillation. Catheter ablation at the left atrial-pulmonary vein junction serves to electrically isolate the pulmonary veins, thereby trapping these foci that are now unable to excite the left atrium.
Other causes of AF must be eliminated by your physician, including hyperthyroidism, recreational drugs (including cocaine), and Wolff-Parkinson-White syndrome (WPW). Alcohol consumption, particularly in excessive amounts, has also been associated with the development of AF and patients should be advised to avoid alcohol intake.
Symptoms
Episodes of atrial fibrillation can lead to rapid heartbeats with symptoms of palpitations and chest fluttering or tremoring. During AF, there is a loss of the simultaneous contraction of the atrial chambers, with loss of its contribution to cardiac output. As a result, patients may feel short of breath, fatigue, dizziness, or rarely, even fainting spells. However, some people with atrial fibrillation may not have any symptoms at all. Episodes of atrial fibrillation can last anywhere from minutes to days or months. Patients with sustained rapid heartbeats should seek medical attention.
Diagnosis
Atrial fibrillation can be diagnosed by your physician via an electrocardiogram or an Ambulatory monitoring device, i.e. Holter or Event monitor, specifically during an arrhythmia episode. Some episodes of atrial fibrillation are intermittent (also referred to as paroxysmal) and not reliably present on a daily basis. In these situations, your physician may recommend a special ambulatory monitoring device that is patient-triggered, i.e. an Event monitor.
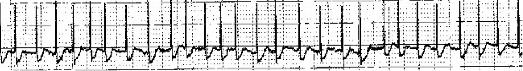
Figure 4. EKG strip of atrial fibrillation with no distinct p-waves (no organized atrial activity) present.
There are three classifications of atrial fibrillation in relation to the duration of the episodes: 1) Paroxysmal, lasting anywhere from seconds to days with typically spontaneous termination, 2) Persistent, where AF does not stop spontaneously and requires medical intervention (either medication or electrical cardioversion) to restore sinus rhythm, and 3) Permanent, where AF is present all of the time and restoration of sinus rhythm is not possible.
Prognosis
Atrial fibrillation can lead to intolerable paroxysms of uncomfortable symptoms for its affected patients. This response is variable, with some patients with no awareness of their symptoms. While most evidence does not point to an increased long-term mortality in those patients placed on appropriate anticoagulation therapy, the long-term morbidity is clearly increased. This morbidity is from the symptoms during AF, the consequences on cardiac structure from prolonged episodes of AF, the small but persistent risk of stroke from AF despite anticoagulation therapy, and the complications of medical interventions to prevent or control the episodes (i.e, bleeding from the anticoagulants, fatigue due to AV nodal agents, and risks of proarrhythmia from antiarrhythmic medications).
Atrial fibrillation is a risk factor for stroke. This is a direct result of the rapid atrial contraction that leads to a stasis or pooling of blood in the atria; particularly, the left atrium. This pooled blood is more likely to form clots that can travel to the systemic circulation and the brain, leading to a cerebrovascular accident or stroke. The risk of stroke is not the same for all people with atrial fibrillation. Therefore, consultation with your physician is very important in determining the presence of additional risk factors for stroke that may require treatment with the blood thinner warfarin (also known as coumadin). Some of these risk factors include age, presence of structural heart disease, hypertension, diabetes, history of stroke, valvular heart disease, heart failure, and coronary artery disease. The decision to implement long-term anticoagulation (that is blood thinners) must be individualized after a discussion between you and your treating physician.
In addition to the risk of stroke, prolonged episodes of AF can cause irreversible changes to the atria, including negative remodeling with atrial enlargement and weakness (myopathy). In addition, prolonged episodes can make reversion and maintenance of normal sinus rhythm more difficult. Long-lasting atrial fibrillation with continuously rapid ventricular rates (heart rates typically greater than 100 beats per min) may cause a tachycardia-induced cardiomyopathy (weakening of ventricular muscle and heart function) and symptoms of congestive heart failure. Atrial fibrillationand atrial flutter tend to coexist, with the significance of atrial flutter in the initiation and perpetuation of atrial fibrillation recently becoming more apparent. It is not uncommon to find patients who have frequent paroxysms of atrial fibrillation post radiofrequency catheter ablation of atrial flutter. Eradication of atrial flutter may or may not improve the burden of atrial fibrillation in these patients. In addition, patients with atrial fibrillation who are treated with antiarrhythmic medications or post catheter ablation with pulmonary vein isolation (discussed below) are noted to revert to atrial flutter. In these situations, atrial flutter can be approached with a catheter ablationprocedure, with eradication of the patient’s atrial tachyarrhythmias.
Therapy
Atrial fibrillation is one of the most difficult cardiac arrhythmias to treat successfully. There are many treatment options, all with inherent risks. The treatment option chosen should be done after careful consultation with your physician given the severity of your symptoms, the compromise to your quality of life, and your coexistent medical conditions. Some patients, especially those with limited symptoms, may desire no treatment and may remain in AF. Other patients may desire to maintain sinus rhythm at all costs due to intolerable symptoms during paroxysms (episodes) of AF. In general, medical therapy is first-line with invasive procedures reserved for patients who have failed or cannot tolerate medical therapy. In some patients, all attempts to suppress and maintain sinus rhythm may fail, leading to permanent AF. As well, due to its episodic nature, AF tends to recur despite repeat procedures to restore sinus rhythm (i.e, cardioversions) if no tailored therapy is designed to suppress AF and maintain normal sinus rhythm, as with medications.
Therapy for patients suffering from AF, as with atrial flutter has three components:
- Control of the ventricular rate (heart rate)
- Conversion and maintenance of sinus rhythm
- Reduction of subsequent stroke risk (as discussed earlier, with anticoagulation)
 Medications used to control the ventricular rate during atrial fibrillation include b-blockers, calcium channel blockers, and less commonly digoxin. These medications can be administered orally on a routine outpatient basis, or via intravenous route if necessary in the emergency room. Some patients may need a combination of medications from different classes. Patients may experience alleviation of symptoms due to slowing of the ventricular response (heart rate) with the use of b-blockers or calcium-channel blockers that delay electrical conduction through the AV node.
Medications used to control the ventricular rate during atrial fibrillation include b-blockers, calcium channel blockers, and less commonly digoxin. These medications can be administered orally on a routine outpatient basis, or via intravenous route if necessary in the emergency room. Some patients may need a combination of medications from different classes. Patients may experience alleviation of symptoms due to slowing of the ventricular response (heart rate) with the use of b-blockers or calcium-channel blockers that delay electrical conduction through the AV node.
Attempts to convert and maintain sinus rhythm can be approached in one of three ways:
- In-hospital electrical or chemical cardioversion,
- Outpatient antiarrhythmic medication therapy, or
- Catheter ablation procedure.
The presence of comorbidities (coexistent disease processes) in some patients may limit the effectiveness of cardioversion procedures and the ability to maintain normal sinus rhythm. As a result, not every patient is an appropriate candidate for rhythm-control, and your physician may opt to treat with rate-control only.
Electrical cardioversion is a procedure done typically in the hospital, where the patient is brought to a specialized room and briefly sedated to sleep. External defibrillation pads are placed in certain locations on the outside of the patient’s chest, with the delivery a brief electric shock (less than a second). The success rate of converting atrial fibrillation to normal sinus rhythm is very high with this procedure. However, it does not guarantee freedom from future reversion back to atrial fibrillation. Chemical cardioversion can also be performed with the infusion of intravenous antiarrhythmic medications. The success rate of this procedure, in comparison to external electrical cardioversion, can be lower especially in patients with prolonged episodes of atrial fibrillation. In addition, some intravenous antiarrhythmics can be contraindicated in subsets of patients with structural heart disease, this limiting their applicability. Electrical or chemical cardioversion in a patient with a prolonged episode of atrial fibrillation (>48 hrs) can only be performed after 4 weeks of documented appropriately therapeutic INRs (measures level of blood anticoagulation) on outpatient warfarin therapy. Treatment with anticoagulation for this 4 week time-frame has been shown to reduce, but not eliminate, the risk of stroke with the cardioversion procedure, by preventing the formation of additional clots in the left atrium. Alternatively, if a cardioversion is more urgent, or if a patient wishes to undergo a more immediate catheter ablation procedure, a transesophageal echocardiogram can be performed to screen for the presence of clots in the left atrial appendage the day of the procedure.
Outpatient oral antiarrhythmic therapy can be instituted in those patients who are typically on oral anticoagulation therapy. They can be helpful in maintaining normal sinus rhythm and prevent recurrences of AF after electrical cardioversion. Your physician may opt to start antiarrhythmic medications in the weeks prior to your cardioversion procedure. There are many antiarrhythmic medications, with only a few requiring a brief hospital stay for initiation. These pharmacologic therapies act directly on the cardiac substrate by slowing electrical conduction in the heart and lengthening the time required for electrical recovery of heart tissue. Drug therapy for AF can be very frustrating for the patient and physician as it can be a “trial and error” proposition; it is not possible to determine ahead of time which medication will work the best in a particular patient. Maintenance of sinus rhythm with complete suppression of atrial fibrillation is typically not possible with medications. Improvements in your quality of life must constantly be reevaluated in relation to the small risks, discomfort, and potential side effects associated with your prescribed treatment regimen. Antiarrhythmic medications are not without risk, as they can increase the incidence of other cardiac arrhythmias.
For those patients who elect to forgo medication either due to
- intolerable symptoms or side effects from their medications,
- recurrent symptoms and episodes despite medical therapy, or
- lack of desire to take daily medications for an extended period of time,
your physician may recommend you undergo an electrophysiology study (EPS) and possible catheter ablation. In brief, these procedures are performed in a special procedure room called an electrophysiology (EP) laboratory. The EP laboratory houses a table and an x-ray (fluoroscopy) machine that is suspended over the table. With the use of specialized electronic and computer equipment, in conjunction with the intracardiac electrode catheters (placed into the heart via the femoral veins) and specialized 3-D electroanatomical mapping systems, your heart rhythm specialist will deliver radiofrequency energy to isolate each of the left atrial pulmonary veins. Radiofrequency energy employed during the catheter ablation procedure heats the tissue enough to destroy the local heart cell function, but without physically cutting through the tissue. The goal of the procedure is to electrically isolate the pulmonary veins which are felt to be the drivers for the initiation and perpetuation of AF. In addition, other areas of potential drivers in the left atrium are targeted for catheter ablation during the procedure to improve the success rate.
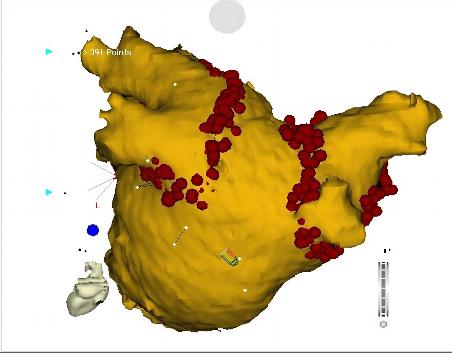
Figure 5. Electroanatomic mapping image during catheter ablation procedure for atrial fibrillation. Pulmonary vein isolation is performed of both the left and right pulmonary veins. Presented here is an image from the posterior, or backside, of the left atrium. The red dots represent sites of delivery of radiofrequency energy by the intracardiac ablation catheter. These ablation lesions are delivered at the junction of the left atrium with pulmonary veins, with the goal of electrically isolating each vein to prevent premature atrial beats and focal atrial tachycardias from the pulmonary veins from entering the left atrium.
The published success rates at different institutions for preventing clinical recurrences in the paroxysmal AF subgroup ranges from 70-80%. These recurrences can be treated with a second catheter ablation procedure. These success rates decrease in the population with permanent chronic AF undergoing catheter ablation procedures. Other contributing atrial tachyarrhythmias, including the earlier discussed AT, AVNRT, atrial flutter, and AVRT may be targeted for ablation, as potential drivers for the initiation of paroxysms of AF.
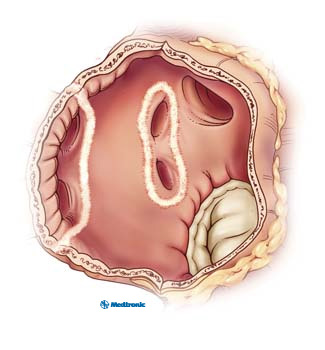
Figure 6. Inside view of the left atrium depicting the isolating lesions delivered by catheter ablation around each set of pulmonary veins.
Video 1. Radiofrequency catheter ablation for atrial fibrillation
Cryoablation, or “freezing therapy” has emerged as an alternative to radiofrequency or “heat energy” in to isolate the pulmonary veins in atrial fibrillation catheter ablation. This technology is just as robust with efficacy outcomes similar to radiofrequency catheter ablation. Please speak with your electrophysiologist to see which energy source for your atrial fibrillation ablation is best fitted for you.
Video 2. Video of cryoablation for pulmonary vein isolation for atrial fibrillation
Not every patient is a candidate for an AF catheter ablation procedure, and an extensive conversation with your physician is important to review the various treatment options with you.
As alluded to earlier, AF may coexist with other atrial tachyarrhythmias, including atrial flutter. Successful catheter ablation of AF may unmask paroxysmal atrial flutter that can be approached with medical versus catheter-based treatments. Withdrawal of anticoagulation therapy is not generally recommended immediately after your catheter ablation procedure for AF. Close monitoring post-ablation with Holters and Event monitors to determine the burden of asymptomatic AF is important. In a subset of patients with no recurrent atrial tachyarrhythmias either clinically or by repeat ambulatory monitoring in the months post-ablation, cautious withdrawal of warfarin therapy can be considered (after 6-12 months), but the long-term data is still limited.
Finally, in patients who are planning to undergo open heart cardiac surgery, an open heart surgical ablation for AF may be appropriate. There are many new techniques being developed utilizing lasers, cryothermia, and alternative energy sources.
Pacemaker Therapy
Patients with sick sinus syndrome and tachycardia-bradycardia syndrome with paroxysms of AF with fast heart rates alternating with symptomatic slow sinus rhythm are candidates for pacemaker implantation. This is a clinical diagnosis reached in consultation with your physician. The pacemaker will prevent symptomatic episodes associated with the slow heart rhythm, including dizziness and loss of consciousness. As well, there is some evidence that the pacing of the topright atrium in these patients may reduce the paroxysms of AF.
Patients with persistent or permanent AF with poor rate control and limited to no ability for rhythm control may be candidates for “ablate and pace” strategy; somewhat of a “last resort” therapy. These patients undergo a catheter-directed ablation of the normal conduction system near the AV node to limit or totally eliminate conduction from the atria to the ventricles, thus becoming 100% dependent on their ventricular pacemaker. This strategy does not eliminate the need for life-long anticoagulation therapy as stroke risk remains the same.




 Silver Spring Office
Silver Spring Office  DC Office (at Providence Hospital)
DC Office (at Providence Hospital)  Hagerstown Office
Hagerstown Office